VIRGINIA, USA — In the past few days, a key FDA panel voted to recommend COVID-19 boosters for Moderna and Johnson & Johnson vaccines.
The committee's advice Friday came three weeks after certain groups who got Pfizer received the green light for a booster dose.
"I'll say within my organization, we have been watching this very closely," said Dr. Kathy Koehl, system director for clinical pharmacy services at Riverside Health System.
Koehl said it's exciting to see the progress surrounding COVID-19 booster doses.
"This is history," she said.
An FDA advisory panel is recommending some who got Moderna to line up for a half-dose booster shot, at least six months after the second dose.
The eligibility requirements for Moderna’s booster would be the same as Pfizer’s.
That means people at least 65 years old, as well as those 18 and up with underlying medical conditions or in high-risk settings.

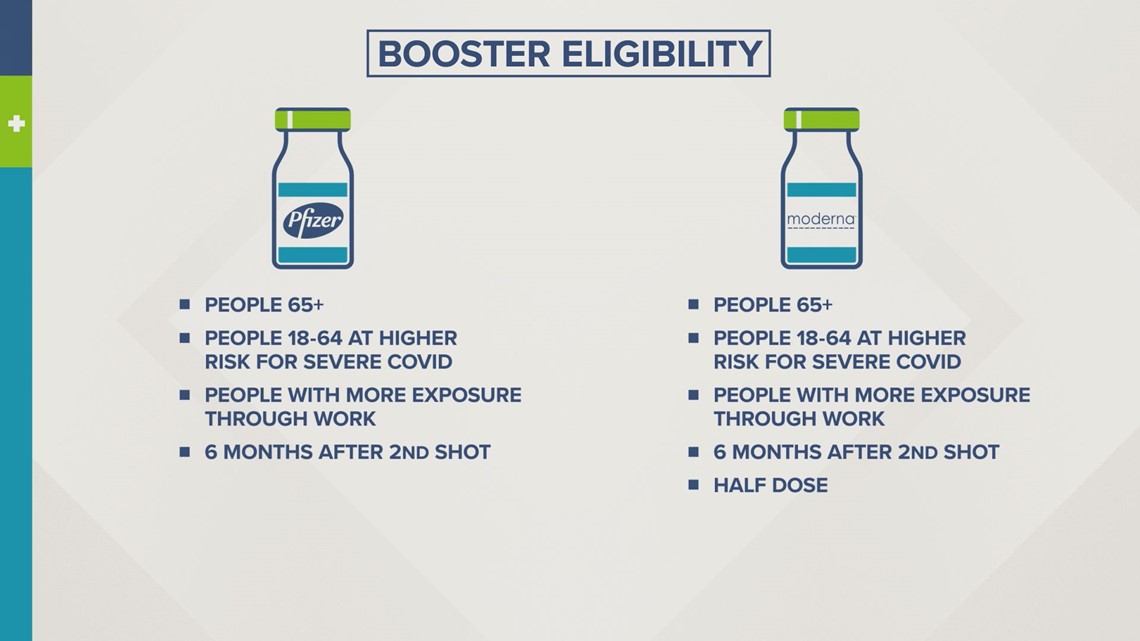
"Currently, what it looks like for the Pfizer vaccine and the Moderna vaccine, is that this is going to help remind our immune system and help get our antibody levels back up to where they were initially," said Dr. Koehl.
Key FDA advisers also recommend another dose of Johnson & Johnson's single-dose vaccine.
This would be for anyone 18 and older, at least two months after the initial shot.

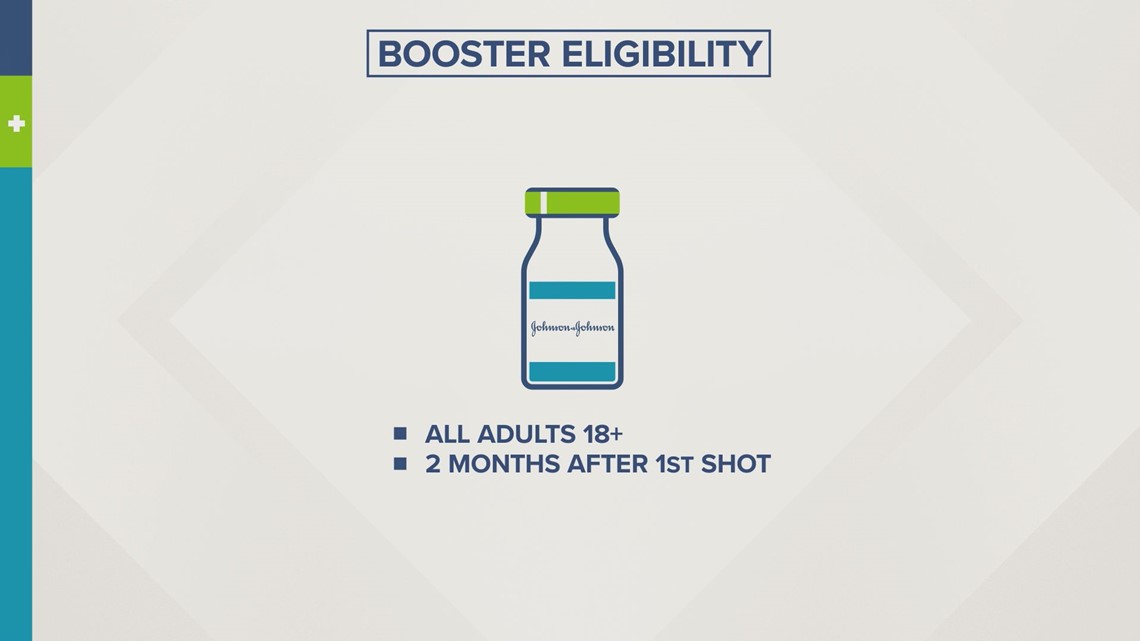
"We get about 70% efficacy right after the vaccine is given. And then months later, we're still seeing about 68% percent efficacy," said Dr. Koehl.
She explained studies about the J&J booster suggest it makes the vaccine more effective overall.
"Once we give that booster dose, we're seeing up to 94% efficacy," said Dr. Koehl.
But with all this news to take in, Dr. Koehl urges the Moderna and J&J crowds to sit tight.
The FDA, as an agency, still needs to approve those boosters. Then, the CDC has to give final approval.
A decision should come next week.
Virginia's vaccine coordinator Dr. Danny Avula released a statement Friday evening calling the latest movements from the FDA a "major step."
He added that VDH teams are working hard to plan booster distribution efforts across the Commonwealth.

